Algem® Aseptic Real-Time Sampling Protocol - ALG App009
algenuity • September 11, 2016
Algae sampling technique for transcriptomics, proteomics, and enzymatic assays
Background
Sampling algal cultures for transcriptomics, proteomics, and enzymatic assays needs to be done quickly and without changing the culturing environment. One common sampling approach is to pause the Algem®, open the Algem® reactor lids, take the Algem® flasks to a laminar flow hood, take the samples, return the flasks to the Algem® reactors, and then resume the Algem® experiment. In most cases, this is a suitable sampling approach.
However, one problem with this sampling approach is that the Algem® flask leaves the controlled light and temperature environment of the Algem® reactor and is temporarily exposed to different conditions. Illumination periods of seconds have been demonstrated to have an effect on gene expression (Huysman 2013). In addition, the sampling approach is somewhat time intensive and limits the possibility of sampling every minute or in smaller unit time series.
Aim
To aseptically sample from the Algem® in real-time, in small unit time series, and without removing the Algem® flasks from their controlled light and temperature environment.
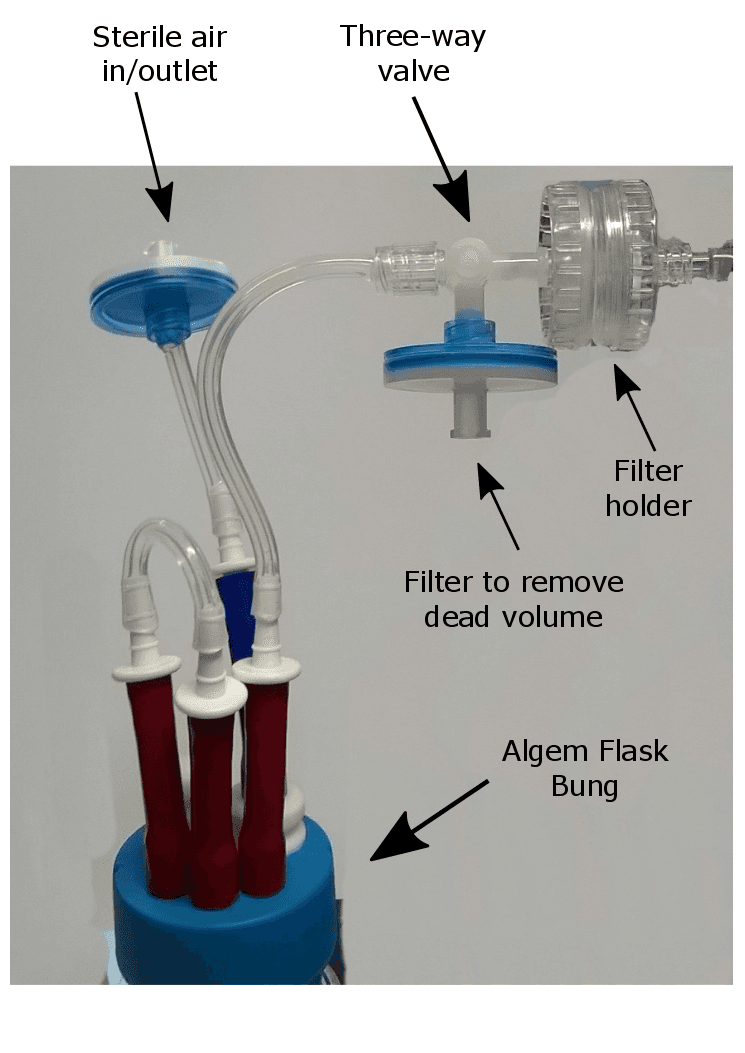
Figure 1 – Algem® flask bung and three-way valve
Method
Before you start an Algem® experiment, take a three-way valve and connect to one of the red inlet tubes on the Algem® flask bung with 1.6mm (inner diameter) silicon tubing (see Figure 1) . The other two red inlets on the Algem® flask bung can be used for introducing gas, media, and/or adjusting pH (in Figure 1, the set-up shows no gas lines for clarity).
Results
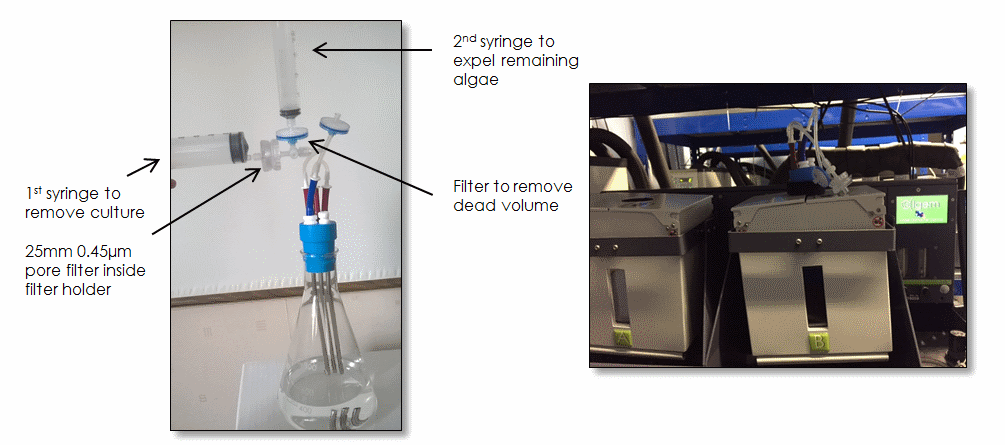
Figure 2 – Algem® flask bung, three-way valve, and syringes Figure 3 – Sampling system in use on Algem®
A 25mm 0.45µm pore PVDF filter is placed inside the filter holder (see Figure 2). Make sure filter is dry. 10 to 25mL of culture is removed from the flask using the first syringe (see Figure 2). Make sure it is one continuous movement. Note that the sample is captured in the filter, not the syringe (See Figures 4 & 5). Alternatively, the filter can be omitted, and the sample can be processed in another way (e.g. quenched in liquid nitrogen).
To remove any remaining algae 'stuck' in the dark zones of the tubing, the three-way valve is opened towards the air filter, and a second syringe is used to inject 10ml of air thereby expelling the algae in the tubes back into the culture vessel (see Figure 2). Once the sampling is complete, the valve is closed.
The method outlined above has also been used to sample algae during the 'night' phase of the diurnal rhythm without exposing the cells to light by using coloured tubing and wrapping the filter disc assembly in tinfoil.
Figure 4 – Opening filter holder to see algae sample on filter Figure 5 – Filter detached from filter holder
Summary
The entire harvest process can be completed in less than 2 minutes without the flask ever having to leave the reactor chamber and without compromising aseptic technique (see Figure 3).
Materials
A sampling pack can be ordered by contacting Algenuity. Materials used are the following:
- Three-way valve - S7521-10EA (Sigma Aldrich)
- Silicon tubing - (Silex - Platinum Cured Silicone Tubing – Translucent 60 degree shore, Wall thickness 1.6mm, Inner diameter 1.6mm, Outer Diameter 4.8mm)
- PVDF filters - P1813-100EA (Sigma Aldrich) to insert inside filter holder (below)
- Polycarbonate syringe filter holder - 25 mm 16517-E (Sartorius)
References
Huysman MJJ, Fortunato AE, Matthijs M, et al. AUREOCHROME1a-Mediated Induction of the Diatom-Specific Cyclin dsCYC2 Controls the Onset of Cell Division in Diatoms (Phaeodactylum tricornutum) . The Plant Cell
. 2013;25(1):215-228.