Culturing a domesticated Chlamydomonas nivalis - ALG App007
algenuity • July 31, 2016
Background
C. nivalis
is a psychrophilic freshwater alga that has adapted to thrive in its own ecological niche within polar and alpine snowfields. The flagellated stages of C. nivalis
enable positional change within the snow layer to achieve the required depth for optimal light and temperature conditions. In nature, C. nivalis
is most frequently found in the encysted stage (hypnoblast) as this is the lifecycle stage most resistant to environmental changes (Remias et al., 2005). This microalga has been observed to tolerate extreme light, low temperatures (2-10°C) and low nutrient conditions (Remias et al., 2010; 2015). When C. nivalis
is cultured under high light and nutrient depletion, it forms mature cysts that are very rigid and difficult to mechanically disrupt (Hoham and Duvel, 2001; Remias et al., 2005). C. nivalis
is of commercial interest due to its high antioxidant and phenolic content (Li et al., 2007), in addition to its ability to produce astaxanthin (Rezanka et al., 2008).
Aim
To determine which medium resulted in the highest growth rate for C. nivalis
at room temperature (20°C); 3N-BBM, HSM and TAP.
Experimental Design
C. nivalis
CCAP 11/128 has been maintained at Algenuity on plate culture on HSM + 15 g/L agar (Chlamydomonas Resource Centre), and in liquid culture with HSM (Chlamydomonas Resource Centre) at 18°C for several years. Preliminary investigations revealed that C. nivalis
was able to tolerate cold conditions as low as 4°C and could be cultured in September Svalbard conditions (modelled on the Algem with low temperatures of 4°C), but growth was very slow. After culturing C. nivalis
over repeated generations it is now able to tolerate conditions much higher than its natural habitat and can grow at room temperature. Initial cultures in late-log grown in HSM were used as the inoculant. For this experiment C. nivalis
was cultured in duplicate with three different media; 3N-BBM+V (CCAP), TAP, and HSM (both recipes from Chlamydomonas Resource Centre) with vitamin B12 addition (0.001 mg/L). Culturing parameters were maintained at 20°C, 150 µmol photons/m²/s, continuous sunlight, 120 rpm with 10 cm³/min aeration with 5 % CO2/air
Results
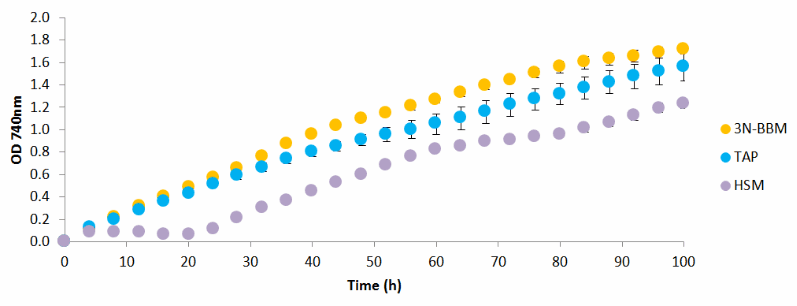
Figure 1 - Growth profile of C. nivalis
cultured in different media (3N-BBM, TAP and HSM) under 20°C, 150 µmol photons/m²/s continuous sunlight, 120 rpm with 10 cm³/min aeration with 5 % CO2
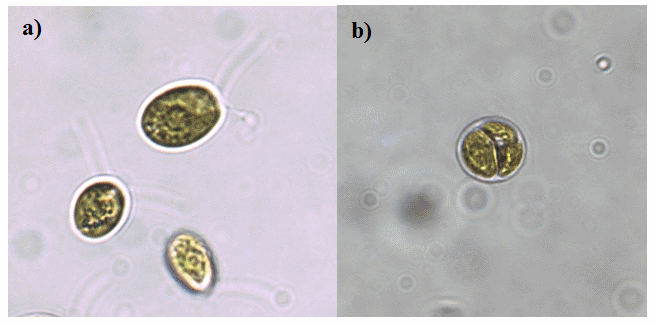
Figure 2 - a) C. nivalis
vegetative cells and b) dividing cells observed throughout the Algem experiment in each medium. No hypnoblasts were observed during the culturing process
Notes
At 20°C C. nivalis
was observed to grow better in 3N-BBM+V and TAP compared with HSM. HSM showed a slow lag time of 24 h before growth was observed. From this experiment it can be deduced that 3N-BBM with the addition of vitamin B12 (cyanocobalamin) is effective for culturing C. nivalis
and should be used for future studies for investigating other parameters for improving growth.
References
Li, H.B., Cheng, K.W., Wong, C.C., Fan, K.W., Chen, F. and Jiang, Y. (2007) Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chemistry
, 102 (3), pp.771-776
Remias, D., Lütz-Meindl, U. and Lütz, C. (2005) Photosynthesis, pigments and ultrastructure of the alpine snow alga Chlamydomonas nivalis. European Journal of Phycology
, 40 (3), pp.259-268
Remias, D., Karsten, U., Lütz, C. and Leya, T. (2010) Physiological and morphological processes in the Alpine snow alga Chloromonas nivalis
(Chlorophyceae) during cyst formation. Protoplasma
, 243 (1- 4), pp.73-86.
Remias, D., Kahr, H. and Jäger, A. (2015) Psychrophilic algae as candidates for outdoor bioreactors in cold countries, 23rd European Biomass Conference and Exhibition, pp. 1911-1912
Řezanka, T., Nedbalová, L., Sigler, K. and Cepák, V. (2008) Identification of astaxanthin diglucoside diesters from snow alga Chlamydomonas nivalis
by liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. Phytochemistry
, 69 (2), pp.479-490.