Culturing a novel strain of Haematococcus pluvialis, LSBB612 - ALG App006
algenuity • July 19, 2016
Background
The genus Haematococcus
is found globally, with reports of isolates from all continents with the exception of Antarctica, with hostile areas of isolation including the artic circle (Klochkova et al., 2013). H. pluvialis
is of commercial interest due to its ability to produce copious amounts of astaxanthin, reaching up to 5 % dry weight in the encysted aplanospore state (Wayame et al., 2013). Astaxanthin is sold as a pigment for aquaculture and in animal feed, and is marketed as an antioxidant for the nutraceutical market. The H. pluvialis
derived astaxanthin industry is commercially successful; however, several constraints are ever-present including issues of contamination and grazing, high extraction costs, high light requirements for encystment, and conversely, photo-bleaching (Shah et al., 2016).
Astaxanthin is produced under high light and nutrient deplete conditions (García-Malea et al., 2008). High temperature is rarely implemented to induce astaxanthin production, as it was reported to severely reduce biomass yield, and thus decrease astaxanthin productivity (Tjahjono et al. 1994). Currently the red stage of astaxanthin production is constrained by biomass production in the green stage, which requires strictly controlled culture conditions. Optimal reported temperatures for the vegetative growth of H. pluvialis
are between 20 and 28°C (Wan et al., 2014), with temperatures in excess of 30°C shown to induce transition from the green vegetative stage to the red stage with the formation of aplanospores. Domínguez-Bocanegra et al., (2004) demonstrated optimal growth at an irradiance of 177 µmol photons/m²/s with higher density cultures achieved under continuous light.
Aim
To demonstrate the growth characteristics of the novel H. pluvialis
strain LSBB612 with an unusually high temperature optimum using the Algem labscale photobioreactor.
Experimental Design
H. pluvialis
LSBB612 starter cultures were grown under 40 µmol photons/m²/s continuous red/blue (3:1) light in TAP ( Chlamydomonas
Resource Centre) + B12 medium (0.001 mg/L cyanocobalamin), with aeration at 15 cm³/min 5% CO₂ in air, shaking at 120 rpm. Algem flasks (1L) containing 400 ml TAP + B12 medium were inoculated at 4 x 10⁴ cells/ml. Flasks were cultured in the Algem in duplicate at 20, 25 or 30°C, 100 µmol photons/m-²/s continuous light (85:15 white/red mix), intermittent aeration, with 5 % CO₂to maintain pH 7, and agitation at 120 rpm.
Results
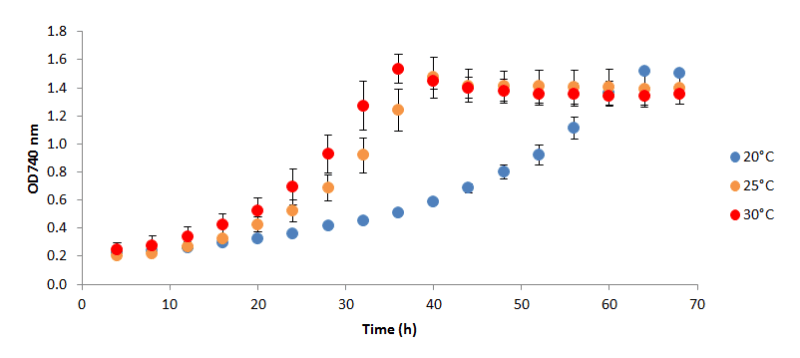
Figure 1 - H. pluvialis
LSBB612 growth under different temperatures (20°C, 25°C and 30°C)
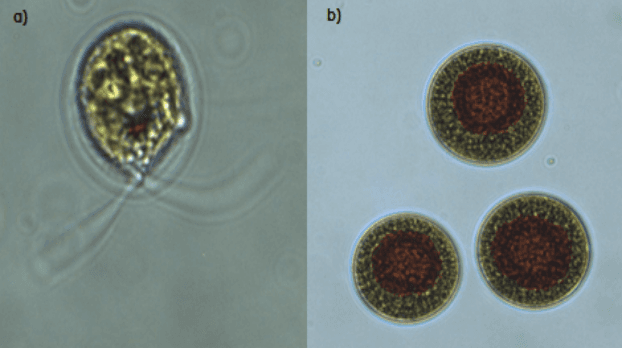
Figure 2 - a) motile macrozooids and b) palmelloids observed during growth
Discussion
H. pluvialis
LSBB612 is classified as non-motile according to the categorisation of Han et al. (2012) as the culture predominates in the palmelloid form rather than the green motile macrozooid. In this experiment it was shown that under the conditions tested, 30°C resulted in the highest growth rate, attaining a maximum doubling time of ~12 h. Growth was shown to plateau for all temperatures at a similar OD due to acetate depletion. This strain of H. pluvialis
offers commercial interest as it is able to tolerate temperatures greater than conventional culture conditions (Wan et al., 2014) and did not encyst during the whole culturing period. A mixture of green motile macrozooids and green palmelloids were maintained. Further experiments will be conducted to ascertain the upper-temperature limit for vegetative growth of this strain.
References
Domínguez-Bocanegra A. R., Guerrero Legarreta I., Martinez Jeronimo F., Tomasini Campocosio A. (2004) Influence of environmental and nutritional factors in the production of astaxanthin from Haematococcus pluvialis . Bioresource Technology
, 92, pp. 209–214
García-Malea, M.C., Sánchez, E. D. R., López, J. C., Fernández, F. A., Sevilla, J. F., Rivas, J., Guerro, M.G. & Grima, E. M. (2006) Comparative analysis of the outdoor culture of Haematococcus pluvialis
in tubular and bubble column photobioreactors, Journal of Biotechnology
, 123 (3), pp. 329-342
Han, D., Wang, J., Sommerfeld, M. & Hu, Q. (2012) Susceptibility and protective mechanisms of motile and non motile cells of Haematococcus pluvialis
(chlorophyceae) to photooxidative stress, Journal of Phycology
, 48 (3), pp. 693-705
Klochkova, T.A., Kwak, M.S., Han, J.W., Motomura, T., Nagasato, C. & Kim, G.H. (2013) Cold-tolerant strain of Haematococcus pluvialis
(Haematococcaceae, Chlorophyta) from Blomstrandhalvøya (Svalbard), Algae
, 28 (2), pp. 185-192
Sarada, R., Bhattacharya, S. & Ravishankar, G. (2002) Optimization of culture conditions for growth of the green alga Haematococcus pluvialis
, World Journal of Microbiology and Biotechnology
, 18 (6), pp. 517-521
Shah, M.M.R., Liang, Y., Cheng, J.J. and Daroch, M. (2016) Astaxanthin-producing green microalga Haematococcus pluvialis
: from single cell to high value commercial products. Frontiers in Plant Science
, 7, p. 531
Tjahjono, A.E., Hayama, Y., Kakizono, T., Terada, Y., Nishio, N. & Nagai, S. (1994) Hyper-accumulation of astaxanthin in a green alga Haematococcus pluvialis
at elevated temperatures, Biotechnology Letters
, 16 (2), pp. 133-138
Wan, M., Hou, D., Li, Y., Fan, J., Huang, J., Liang, S., Wang, W., Pan, R., Wang, J. & Li, S. (2014) The effective photoinduction of Haematococcus pluvialis
for accumulating astaxanthin with attached cultivation, Bioresource Technology
, 163, pp. 26-32
Wayama, M., Ota, S., Matsuura, H., Nango, N., Hirata, A. & Kawano, S. (2013) Three-Dimensional Ultrastructural Study of Oil and Astaxanthin Accumulation during Encystment in the Green Alga Haematococcus pluvialis’ , PLOS One
, 8 (1)