Culturing Galdieria sulphuraria - ALG App004
algenuity • June 15, 2016
Background to G. sulphuraria
G. sulphuraria
is an extremophilic, spherical, spore-forming red alga commonly found in hot acid springs. It is an acidophilic and thermophilic alga which grows phototrophically and mixotrophically, and is capable of heterotrophic growth on sugars, alcohols and amino acids (Gross and Schnarrenberger, 1995; Oesterhelt and Gross, 2002; Barbier et al., 2005). G. sulphuraria
has commercial potential for wastewater remediation (Schönknecht et al., 2013; Selvaratnem et al., 2014) and the mass production of the phycobiliprotein phycocyanin (Schmidt et al., 2005).
Aim
To confirm whether G. sulphuraria
(SAG 107.79) can tolerate high temperatures (50°C) and acidic (pH 4) conditions, and to observe how light intensity and photoperiod affect growth.
Experimental Design
Two experiments were conducted, one investigating the effects of temperature, and the other investigating photoperiod and light intensity. Growth comparisons were made based on hourly optical density (OD) measurements at 740nm.
For both experiments exponentially growing cultures of G. sulphuraria
in late-log phase were harvested and inoculated at 5 x 10⁵ cells/ml into 1 L flasks with 400 ml Cyanidium medium (SAG) + 150 mM glucose (pH 4). Soil extract was replaced by 1 ml/L Special K trace elements according to (Kropat and Malasarn, 2010). G. sulphuraria
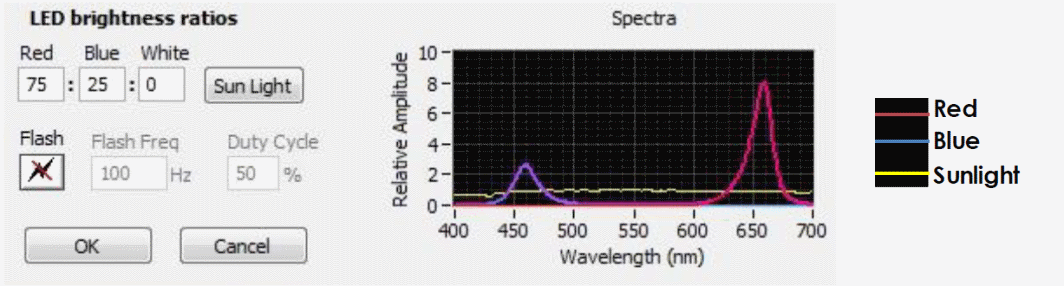
was cultured with red and blue light as indicated in Figure 1; preliminary experiments having revealed that red and blue light combined at the ratio stated resulted in better growth than white light. Flasks were mixed at 90 rpm without aeration.
To investigate temperature cultures were incubated at 25°C, 40°C, and 50°C under continuous light at 100 μmol photons/m²/s. Investigations into photoperiods and light intensity were conducted at 50°C under either a 12:12 photoperiod or continuous light, both at light intensities of 100 and 200 μmol photons/m²/s.
Figure 1 - LED spectra for G. sulphuraria cultivation with peaks included in the blue (450-500 nm) and red regions (610-700)
Results
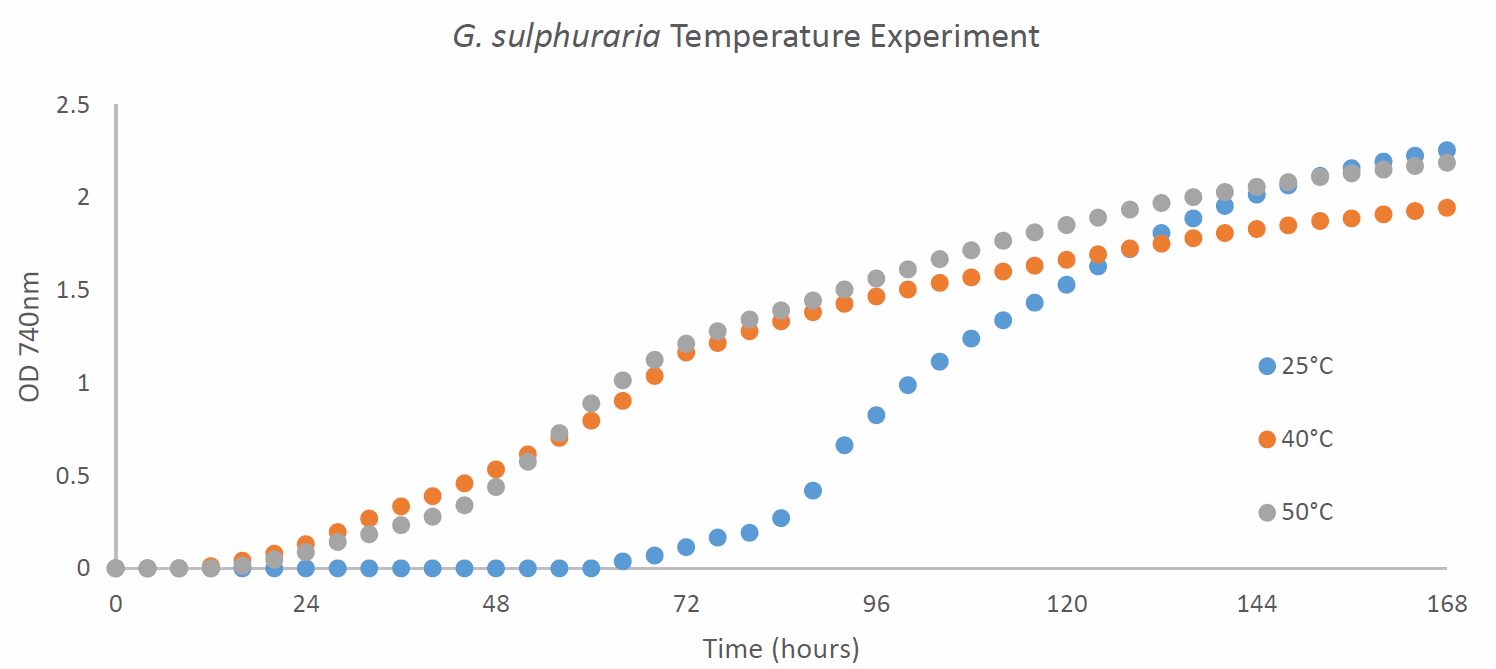
Figure 2 - Growth profile of G. sulphuraria cultured mixotrophically in Cyanidium medium with glucose at 100 μmol photons/m²/s continuous light at different temperatures (25, 40 and 50°C) with red: blue light at a ratio of 3:1
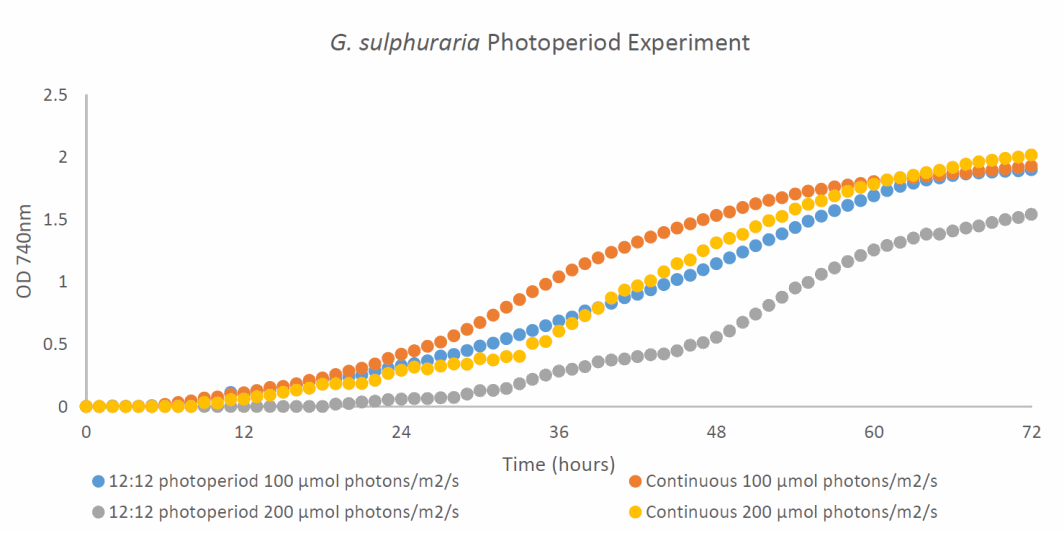
Figure 3 - Growth profile of G. sulphuraria cultured mixotrophically in Cyanidium medium with glucose at 50°C under different photoperiods (12:12 photoperiod and continuous) and light intensities (100 μmol photons/m²/s and 200 μmol photons with red: blue light at 3:1)
Discussion
G. sulphuraria
was observed to grow optimally at 50°C with a similar growth profile at 40°C but a slightly lower final OD 740 nm maximum (Figure 2). There was a long lag when G. sulphuraria
was cultured at 25°C (Figure 2). G. sulphuraria
appears to not just be thermotolerant but thermophilic and acidophilic. G. sulphuraria
grew better under the lower light intensity of 100 μmol photons/m²/s with similar growth patterns under a 12:12 photoperiod and continuous light under the conditions tested (Figure 3). A 12:12 photoperiod under 200 μmol photons/m²/s resulted in the poorest growth. Future experiments should focus on culturing G. sulphuraria
at high temperatures comparing photoautrophic media with heterotrophic conditions where G. sulphuraria has been observed to have a higher doubling time (Graziani et al., 2013).
References
Barbier, G., Oesterhelt, C., Larson, M.D., Halgren, R.G., Wilkerson, C., Garavito, R.M., Benning, C. and Weber, A.P. (2005) Comparative genomics of two closely related unicellular thermo-acidophilic red algae, Galdieria sulphuraria
and Cyanidioschyzon merolae
, reveals the molecular basis of the metabolic flexibility of Galdieria sulphuraria
and significant differences in carbohydrate metabolism of both algae. Plant physiology
, 137 (2), pp. 460-474.
Graziani, G., Schiavo, S., Nicolai, M.A., Buono, S., Fogliano, V., Pinto, G. and Pollio, A. (2013) Microalgae as human food: chemical and nutritional characteristics of the thermo-acidophilic microalga Galdieria sulphuraria
. Food and Function
, 4 (1), pp.144-152
.
Gross, W. and Schnarrenberger, C. (1995) Heterotrophic growth of two strains of the acido-thermophilic red alga Galdieria sulphuraria . Plant and Cell Physiology
, 36 (4), pp. 633-638.
Kropat, J and Malasarn, D (2010) Merchant Lab Research Resources: Special K Traces. Available at: http://www.chem.ucla.edu/dept/Faculty/merchant/pdf/SpecialKTraceElementsJune2.pdf . Accessed: 21/04/16.
Oesterhelt, C. and Gross, W. (2002) Different Sugar Kinases Are Involved in the Sugar Sensing of Galdieria sulphuraria . Plant physiology
, 128 (1), pp. 291-299.
Schmidt, R.A., Wiebe, M.G. and Eriksen, N.T. (2005) Heterotrophic high cell‐density fed‐batch cultures of the phycocyanin‐producing red alga Galdieria sulphuraria
. Biotechnology and bioengineering
, 90(1), pp.77- 84.
Schönknecht, G., Chen, W.H., Ternes, C.M., Barbier, G.G., Shrestha, R.P., Stanke, M., Bräutigam, A., Baker, B.J., Banfield, J.F., Garavito, R.M. and Carr, K. (2013) Gene transfer from bacteria and archaea facilitated evolution of an extremophilic eukaryote. Science
, 339(6124), pp.1207-1210.
Selvaratnam, T., Pegallapati, A.K., Montelya, F., Rodriguez, G., Nirmalakhandan, N., Van Voorhies, W. and Lammers, P.J. (2014) Evaluation of a thermo-tolerant acidophilic alga, Galdieria sulphuraria , for nutrient removal from urban wastewaters. Bioresource Technology
, 156, pp.395-399.